The coronavirus pandemic has compelled regulatory bodies, such as the FDA, to ease their regulatory norms for fetal monitors to support the uninterrupted care regime of expectant mothers. Relaxations in rules have paved the way for several key players to smoothly launch fetal monitors equipped with tele-ultrasound monitoring, noninvasive features, and portable technologies.
The FDA’s enforcement policy for noninvasive fetal and maternal monitoring devices aims to increase access to devices that could reduce the need for in-clinic visits and reduce the burden on doctor’s offices and hospitals during the pandemic.
The fetal monitoring market is expanding with the latest innovative technologies, backed by escalating research and product launches. Fetal monitoring technologies are now being streamlined to provide greater assistance in utero fetal surgeries, consisting of challenging physical fetal access and technological complications.
Download PDF Brochure With Latest Edition @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=35700261
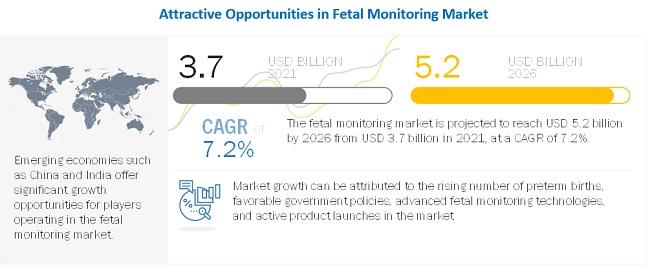
Fetal cardiac assessments have also witnessed progress that supports high-fidelity hemodynamic and continuous physiologic monitoring, thus enabling early diagnosis and treatment.
Computer-Aided Decision Support Systems and Artificial Intelligence (AI) are also utilized in continuous cardiotocography (CTG) or fetal heart rate (FHR) auscultation during labor. The patient’s electronic health records (EHRs) are utilized along with computational methods, machine learning, and deep learning tools.
Fetal and neonatal assessments are carried out during antepartum via percutaneous umbilical cord blood sampling and during intrapartum with fetal scalp blood sampling immediately after birth. Fetal phonocardiography has been integrated with advanced data acquisition systems and databases. Progressing data analytics, synthetic data generation by advanced mathematical models, and classifications & processing algorithms are under active research & development to increase fetal motoring data processing efficacy.
The SonoScape system from GE Healthcare costs around USD 9,000. These machines also incur maintenance charges, which amount to 15% of the unit’s purchasing cost. The high costs of fetal monitoring equipment can be challenging to low-income nations and low-resource hospitals, thus restraining this market’s growth.
Key Market Players
The major players operating in fetal monitoring market are Cardinal Health, Inc. (US), Koninklijke Philips N.V. (Netherlands), GE Healthcare (US), Siemens Healthineers (Germany), FUJIFILM SonoSite, Inc. (US), Natus Medical Incorporated (US), Huntleigh Healthcare Limited (UK), The Cooper Companies Inc. (US), CONTEC Medical Systems Co., Ltd. (China), EDAN Diagnostics, Inc. (China), Neoventa Medical AB (Sweden), Bionet Co., Ltd. (South Korea), Progetti Srl (Italy), TRISMED Co., Ltd. (Republic of Korea)